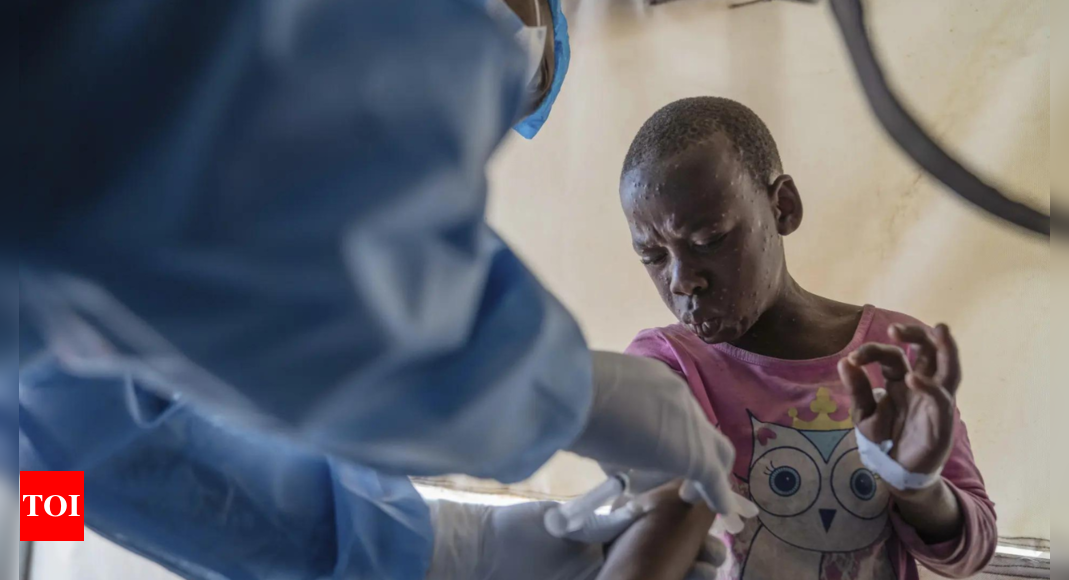
The World Health Organisation (WHO) announced on Friday that it has granted its first authorisation for the use of a vaccine against mpox in adults. This marks a significant step in the fight against the disease, particularly in Africa, where mpox has had a notable impact.
The vaccine, produced by Bavarian Nordic A/S, can now be purchased by donors such as Gavi and Unicef.However, supplies are limited since there is only one manufacturer.
“This first (authorisation) of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future,” said WHO Director-General Tedros Adhanom Ghebreyesus.
WHO is establishing a system to ensure fair distribution of mpox tests, treatments, and vaccines to countries in need.
The approved two-dose vaccine is for those aged 18 and above, but may also be used for younger individuals in outbreak situations where the benefits outweigh the risks.
The Bavarian Nordic vaccine was previously approved in Europe and North America during the 2022 mpox outbreak. While it has helped reduce the spread among adults, its effectiveness in children is not well documented.
Most cases of mpox in Congo, the hardest-hit country, involve children under 15, who also have the highest death rates from the disease.
Globally, over 103,000 mpox cases have been confirmed in more than 120 countries since the outbreak began two years ago. WHO reported that 723 deaths have occurred in over a dozen African countries.
African health experts estimate the continent needs about 10 million vaccines to control the outbreaks, but donor countries have only pledged about 3.6 million. As of last week, Congo had received 250,000 doses.
Recently, the Africa CDC reported 107 new deaths and over 3,000 new cases in one week, following the launch of a continent-wide response plan.
Mpox is related to smallpox but tends to cause milder symptoms like fever, chills, and body aches. Severe cases can result in lesions on the face, hands, chest, and genitals.
The vaccine, produced by Bavarian Nordic A/S, can now be purchased by donors such as Gavi and Unicef.However, supplies are limited since there is only one manufacturer.
“This first (authorisation) of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future,” said WHO Director-General Tedros Adhanom Ghebreyesus.
WHO is establishing a system to ensure fair distribution of mpox tests, treatments, and vaccines to countries in need.
The approved two-dose vaccine is for those aged 18 and above, but may also be used for younger individuals in outbreak situations where the benefits outweigh the risks.
The Bavarian Nordic vaccine was previously approved in Europe and North America during the 2022 mpox outbreak. While it has helped reduce the spread among adults, its effectiveness in children is not well documented.
Most cases of mpox in Congo, the hardest-hit country, involve children under 15, who also have the highest death rates from the disease.
Globally, over 103,000 mpox cases have been confirmed in more than 120 countries since the outbreak began two years ago. WHO reported that 723 deaths have occurred in over a dozen African countries.
African health experts estimate the continent needs about 10 million vaccines to control the outbreaks, but donor countries have only pledged about 3.6 million. As of last week, Congo had received 250,000 doses.
Recently, the Africa CDC reported 107 new deaths and over 3,000 new cases in one week, following the launch of a continent-wide response plan.
Mpox is related to smallpox but tends to cause milder symptoms like fever, chills, and body aches. Severe cases can result in lesions on the face, hands, chest, and genitals.